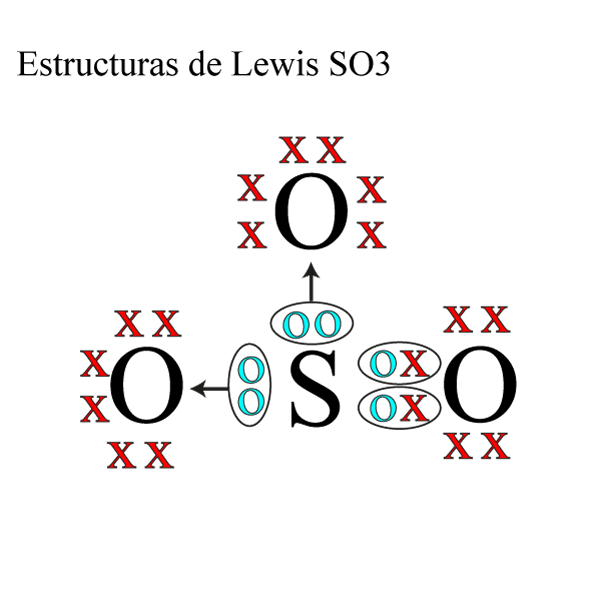
Estructura de Lewis SO3 » Quimica Online
The SO3 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the SO3 molecule.

Draw The Lewis Dot Structure For So3 2 slidesharedocs
Lewis Structure of SO3 (Sulfur Trioxide) chemistNATE 260K subscribers Subscribe Subscribed 628K views 9 years ago Lewis Structures How to draw the Lewis Structure of SO3 (sulfur trioxide).

SO3 Lewis StructureLewis structure of SO3 (Sulfur trioxide) YouTube
SO3 Lewis Structure - Sulfur Trioxide The Organic Chemistry Tutor 7.24M subscribers Subscribe Subscribed 607 70K views 3 years ago This chemistry video explains how to draw the Lewis.
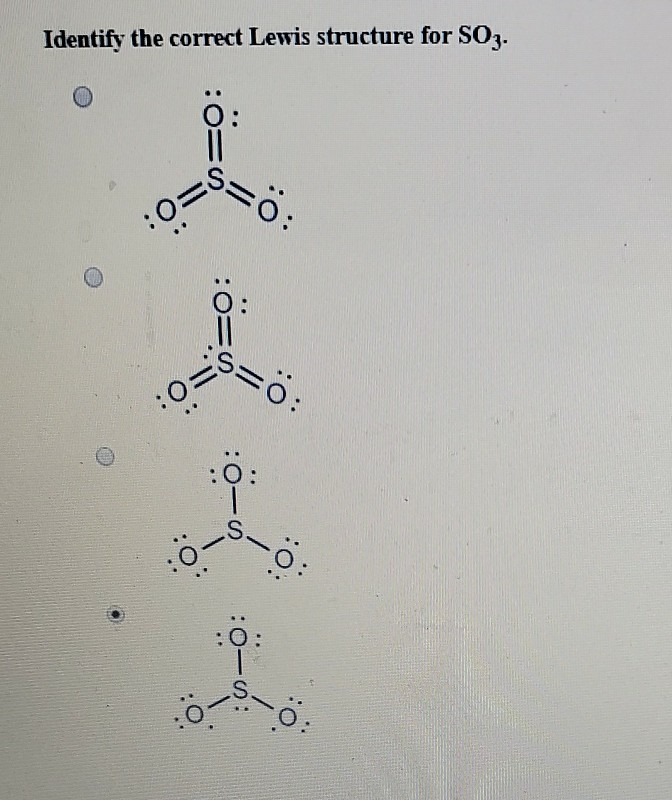
Solved Identify the correct Lewis structure for SO3.
Sulfur trioxide (alternative spelling sulphur trioxide, also known as nisso sulfan) is the chemical compound with the formula SO 3. It has been described as "unquestionably the most important economically" sulfur oxide. [1] It is prepared on an industrial scale as a precursor to sulfuric acid .

Image Showing Resonance Strcuture Of So3 So3 Resonance Structures
Sulfur Trioxide Molecular Geometry Being an intelligent and well-practiced human being, you must know what is molecular geometry, but let me revise it for the all young students out there. Molecular geometry is the three-dimensional structure of the atoms which helps in the constitution of a molecule.

How to draw SO3 Lewis Structure? Science Education and Tutorials
-the physical properties of a molecule such as boiling point, surface tension, etc. Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3.

Lewis Dot Structure for SO3 (Sulfur trioxide) YouTube
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and.

SO3 Lewis Structure, Molecular Geometry, and Hybridization
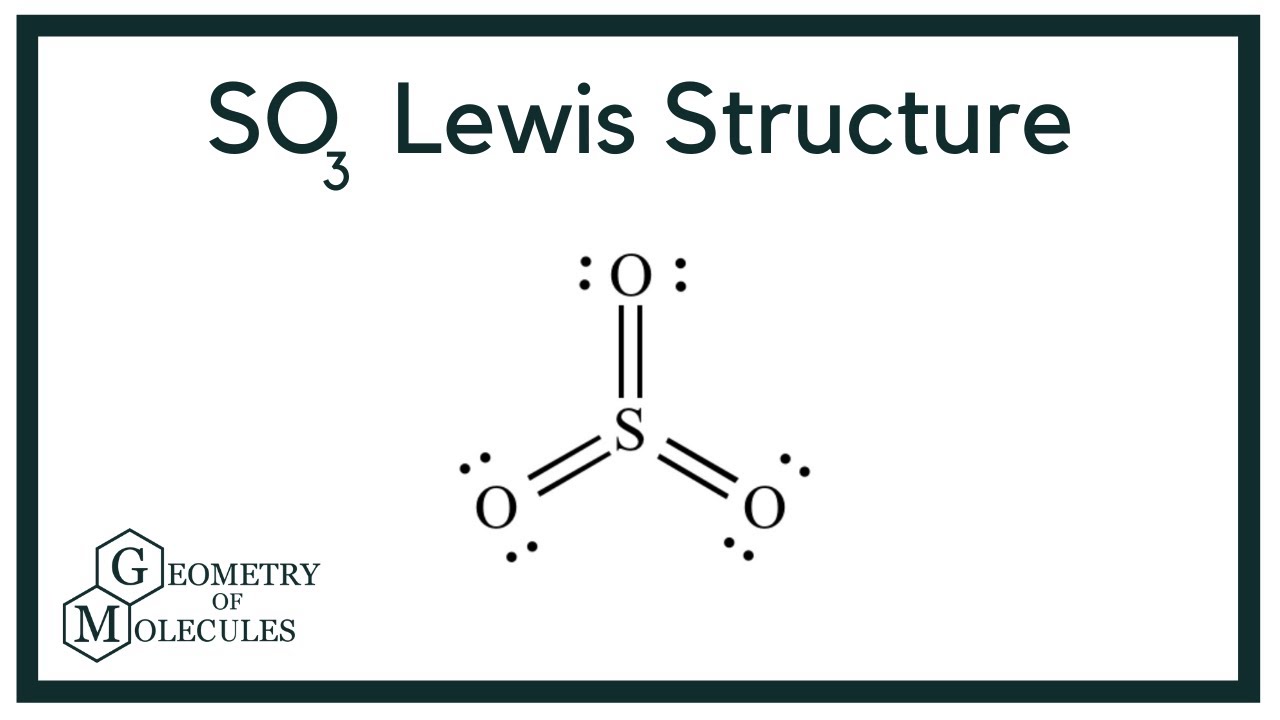
The SO3 Lewis structure shows a central Sulfur (S) atom with three Oxygen (O) atoms around it. These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. In this page, you'll find a detailed, step-by-step guide on how to draw the Lewis structure for SO3. Step-by-Step Guide to […]
SO3 Lewis Structure (Sulfur Trioxide) YouTube
0:00 / 2:55 SO3 2- Lewis Structure - How to Draw the Lewis Structure for SO3 2- (Sulfite Ion) Wayne Breslyn 724K subscribers Join Subscribe Subscribed 1.9K Share 411K views 10 years ago.
LEWIS DIAGRAM FOR SO3 YouTube
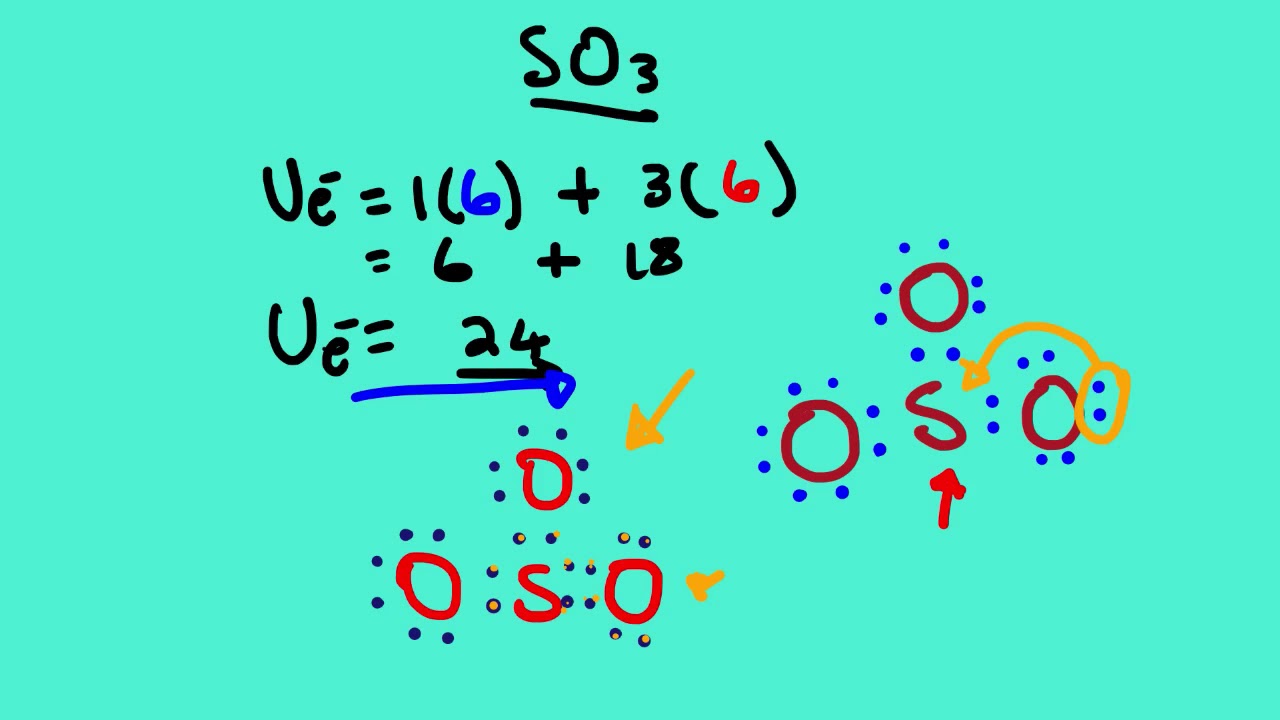
Let's do the SO3 Lewis structure. Sulfur has 6 valence electrons. Oxygen has 6, but we've got three Oxygens, for a total of; 6 plus 18; 24 valence electrons. Let's put Sulfur at the center and then the Oxygens around the outside, all three of them. Now we'll put two valence electrons between each atom to form a chemical bond.

Estructura de Lewis SO3 » Quimica Online
1.3K 357K views 10 years ago SO3 Lewis, Shape, Hybridization, Polarity, and more. A step-by-step explanation of how to draw the SO3 Lewis Dot Structure (Sulfur trioxide). For the SO3.

So3 Lewis Structure With Formal Charges
The SO3 Lewis structure shows a central Sulfur (S) atom with three Oxygen (O) atoms around it. These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. In this page, you'll find a detailed, step-by-step guide on how to draw the Lewis structure for SO3. Step-by-Step Guide to Drawing the Lewis Structure of SO3

make structure of hybridization of SO3 Brainly.in
Steps of drawing SO3 lewis structure Step 1: Find the total valence electrons in SO3 molecule. In order to find the total valence electrons in SO3 (sulfur trioxide) molecule, first of all you should know the valence electrons present in sulfur atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

SO3 Molecular Geometry / Shape and Bond Angles (Sulfur Trioxide) YouTube
Lewis structure of SO3 (or Sulfur trioxide) contains three double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center and it is surrounded by 3 Oxygen atoms (O). The Sulfur atom does not have a lone pair while all the three Oxygen atoms have 2 lone pairs.
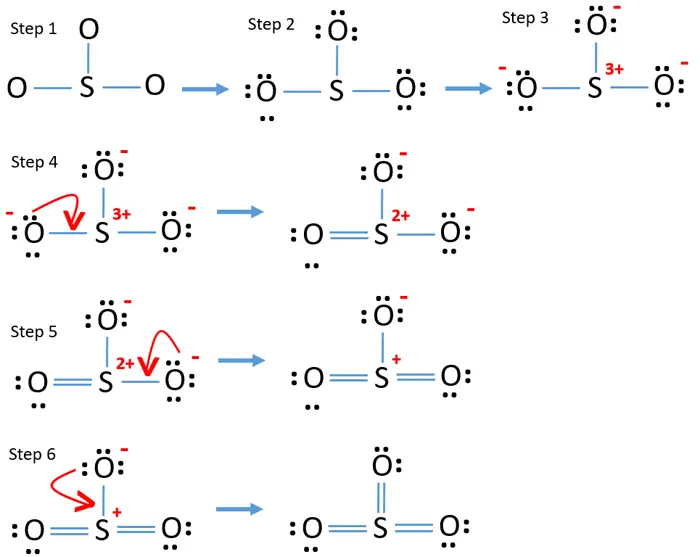
steps of drawing SO3 lewis structure VSEPR method
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

SO3 Lewis Structure, Molecular Geometry, and Hybridization
To draw this structure, begin by sketching a rough diagram of the molecular arrangement. Next, indicate the lone pairs on each atom and check for any formal charges. If formal charges are present, convert lone pairs to minimize these charges. Repeat this process until all charges are minimized.